Refrigerant R22
Refrigerant R22
Before we get ahead of ourselves, R22 will phase out in Australia by 2029. Top-up of R22 equipment beyond 2029 can only be using reclaimed R22. Its price increase would be substantially more expensive. The current retail price of R22 exceeded $200/kg!
Let us continue. The refrigeration industry uses many fluids, called refrigerants, which all evaporate at low temperatures at atmospheric pressure.
These fluids are still sometimes known as “freons”. You might know that the word Freon is the trade name used by the “Dupont” company for these materials. The Elf Atochem group, for example, uses the name “Forane” for these materials. In practice, each manufacturer gives their refrigerants a trading name.

All these refrigerants are sold and marketed in a series of different size containers, exactly like the cylinders of butane or propane that you may have had occasion to use for cooking or heating.
To avoid getting lost amongst the different brand names, we identify refrigerants by the letter R (R for Refrigerant).
For example, in the years gone by, R22 has been one of the refrigerants most commonly used in air conditioning and is the one we’ll be using as an example throughout. R22 is known by such names as Freon 22, Forane 22, Suva 22, etc., according to the manufacturer.
It is crucial to understand that R22 is identical to whoever the manufacturer is. This situation is like buying petrol from Caltex, BP, Shell etc.
We can, therefore, mix Freon 22 with Forane 22. For example, the two materials are identical.
R22 is particularly interesting as it evaporates at -42°C at atmospheric pressure (compared with 100°C for water, 35°C for ether or 300°C for cooking oil).
Because of its low evaporating temperature, R22 allows us to easily obtain a temperature of less than 0°C in our fridge’s freezer. It is our objective.
The pressure-temperature relationship for refrigerant R22 follows the same general rules as that of water. The lower the pressure above the liquid, the lower the evaporation temperature (and vice versa).
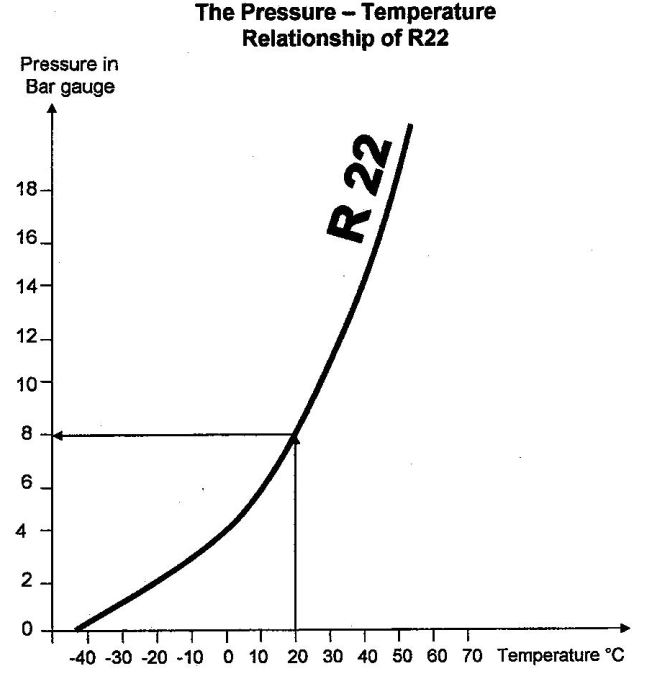
To give you a feeling for this, the diagram below shows you the pressure-temperature relationship for R22 from -40°C to +20°C.
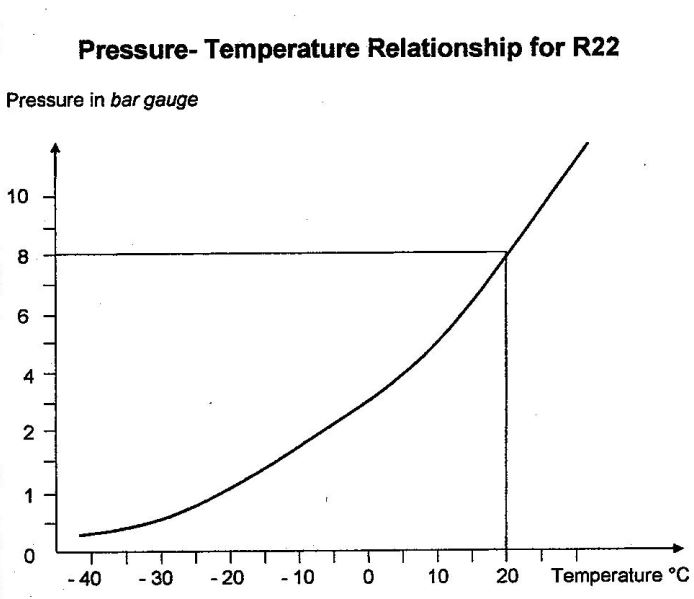
You will see from the diagram that at 0 bar gauge (that is, at atmospheric pressure), the evaporation temperature of R22 is -42°C. However, at 8 bar, its evaporation temperature rises to about 20°C.
You can see from this diagram as the evaporation pressure increases, the evaporation temperature correspondingly increases. So you should know why. Remember the explanation involving internal and external pressure?
Thus, the evaporation pressure and temperature of R22 are linked, and they change in the same direction, precisely like water, but with a pressure–temperature relationship involving very different values.
Consider the interior of the cylinder of R22 opposite. There is liquid in the bottom of the container and vapour above it. The internal pressure of the liquid is in equilibrium with the external pressure of the vapour. If the temperature of the cylinder increases, the refrigerant pressure also increases in line with the pressure-temperature relationship. The resulting internal pressure is 8 bar at 20°C, 11 bar at 30°C, 14 bar at 40°C and 19 bar at 50°C.
So it’s better to avoid storing refrigerant cylinders in direct sunlight or close to a heat source to prevent dangerous over-pressurisation.


We know from experience that some readers will find it difficult to visualise how a refrigerant like R22 is a liquid that can evaporate at -42°C at atmospheric pressure!
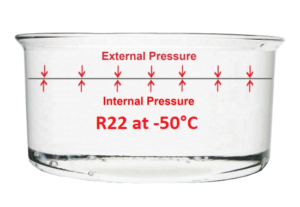
To help us shed light on this phenomenon, let’s imagine ourselves inside a cold room Where the temperature is -50°C, or even at the North Pole in the middle of winter. Under these conditions, it will be possible to pour R22 into a saucepan, which will remain entirely liquid.
What happens is that the temperature of the R22 being at minus 50°C, the internal forces are weaker than the external forces, which correspond to atmospheric pressure. As a result, the surface of the liquid contained in the saucepan remains flat and still.
To make the R22 evaporate, we only need to heat it on a burner, increasing the internal forces. Then, when the temperature reaches -42°C, evaporation will begin.
It would help if you remembered that under these same conditions, water evaporates at 100°C since the external forces correspond to atmospheric pressure.
But where can we find R22, and how can we keep it liquid if it evaporates at -42°C?
Take butane, for example. Do you know the evaporation temperature of butane gas? At atmospheric pressure, it evaporates at -0.5°C. You will have undoubtedly come across butane before.


Commercial butane for heating and cooking is in cylinders. Each cylinder holds a mixture of liquid and gaseous butane. If the cylinder only contained butane in the vapour state, it would need changing much too often! The temperature of this mixture isn’t always at 0.5°C. Generally, the temperature of the cylinder is the same as its surrounding. For example, if it’s 25°C outside the cylinder, the butane is also at 25°C on the inside.
There is a mixture of liquid butane (at the bottom) and gaseous butane (at the top). But shouldn’t the butane be completely vaporised at 25°C? What do you think?
Let’s examine the cylinder of R22 opposite. It’s nearly identical to the cylinder of butane that we’ve just covered, but it also has a dip tube.
This tube allows us to draw liquid R22 from the bottom of the cylinder. In contrast, we use butane or propane in its gaseous forms. We are more interested in R22 in its liquid form.
But what exactly is happening in this cylinder?
There is a mixture of liquid R22 (at the bottom) and gaseous R22 (at the top). But, at 25°C, shouldn’t all the R22 be vaporised? What do you think?
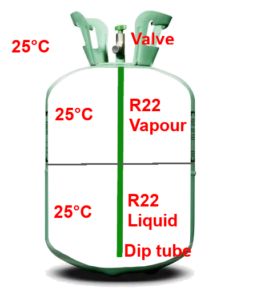
R22 is also in cylinders similar to those used for butane or propane.
These cylinders are just like pressure cookers without safety valves. The energy from the surrounding temperature causes some of the R22 in the cylinder to evaporate. Evaporation will occur until the equilibrium between the internal and external forces is again.
However, unlike the pressure cooker, there is no safety valve to limit the pressure developed inside the cylinder.
In this case, when the surrounding temperature increases, what will happen to the pressure of R22 inside the cylinder? Will it increase, decrease or remain constant?
It’s the same as water, and the pressure-temperature relationship plays a part because R22 obeys the same laws of nature as other fluids: its pressure varies with temperature.
But we still need to answer the question!
It is not essential to memorise all the values in the pressure-temperature relationship of R22. However, if we want to maintain a fridge system, we must be able to understand it in general terms at least!
Let’s look again at the pressure-temperature relationship of R22.
Let’s assume that the ambient temperature is 20°C for the cylinder’s surroundings. The R22 will also be at 20°C, and its pressure will therefore be 8 bar, as shown in the graph.
You can use the graph to verify that at atmospheric pressure, the evaporation temperature of R22 is -42°C. Furthermore, you can see (just as with water, butane and propane) that as the temperature of the R22 increases, the pressure increases, and vice versa.
In that case, how do we measure pressure?
We use a pressure gauge to measure pressure. It is one of the essential tools of the refrigeration engineer. A pressure gauge makes it easier to check a system’s correct operation effectively. A refrigeration gauge shows a refrigeration system’s operating pressures and temperatures.
Wait a minute! A pressure gauge lets you measure pressure, but how does a pressure gauge measure temperature? Wouldn’t you use a thermometer?
You could use a thermometer to measure a temperature, and the pressure gauge is a device that allows you to measure pressure. However, refrigeration engineers’ pressure gauges are special. They have a pressure scale and also a temperature scale.
Refrigeration gauges are devices for measuring pressures. However, the dials of these gauges are slightly more complicated as they also show one or more temperatures.
You can see the opposite view of one of the refrigeration engineer’s principal tools: a “set of gauges”, often called a gauge manifold”.

Back to Basic!
Related
Read more: Fan wall
Read more: How to verify the percentage of outside air in an enclosure
Read more: BCA Part J5 Air-conditioning system control
Read more: Microbial Induced Corrosion (MIC) in Pipes
Read more: Is your kitchen exhaust system a fire hazard
Read more: What is coanda effect