The Evaporation of R22
The Evaporation of R22
Let’s take a cylinder of R22 at an ambient temperature of 20°C. It’s filled with a liquid-vapour mixture at 20°C, corresponding to a gauge pressure of 8 bar on the pressure-temperature relationship of R22.
If we open the valve, liquid of 8 bar immediately comes out of the cylinder via the dip tube (there is no way that the vapour, trapped between the layer of liquid and the top of the cylinder to get out).
As it emerges from the cylinder, the molecules of liquid at 8 bar suddenly find themselves exposed to a much lower external pressure of 0 bar gauge. It rapidly causes the R22 to boil (as water does at 100°C) and change into a vapour.
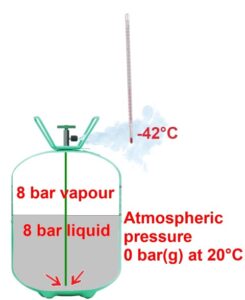
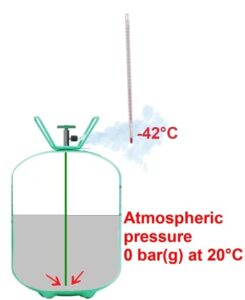
But for the refrigerant to vaporise, the liquid needs heat. It will take this heat from any warmer material that it comes into contact. For example, if we place a thermometer at the cylinder valve outlet, it reads -42°C, which corresponds precisely to the evaporation temperature at atmospheric pressure.


DANGER! DANGER! DANGER! if you plunge your hand at 30°C in boiling water at 100°C, it represents a temperature difference of 70°C. You will scald your hand.
If you touch R22 at -42°C, it will represent a temperature difference of 72°C for your hand, and this will cause as severe a burn as in boiling water.
Of course, if you touch the outside of the cylinder at 20°C, there is no such problem.
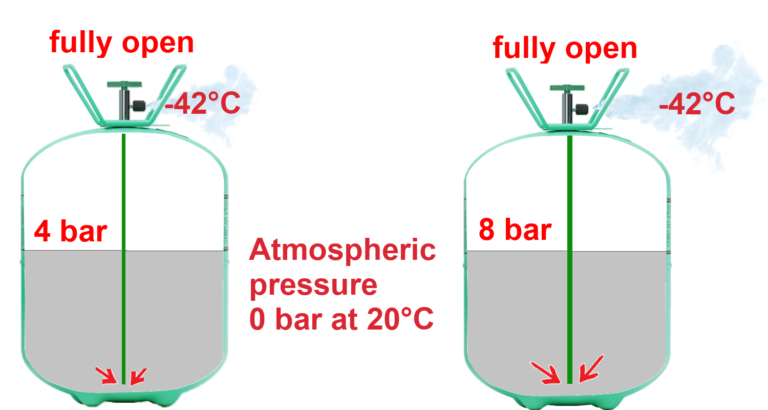
For the same cylinder valve opening, the higher the pressure inside the cylinder, the greater the liquid flow.
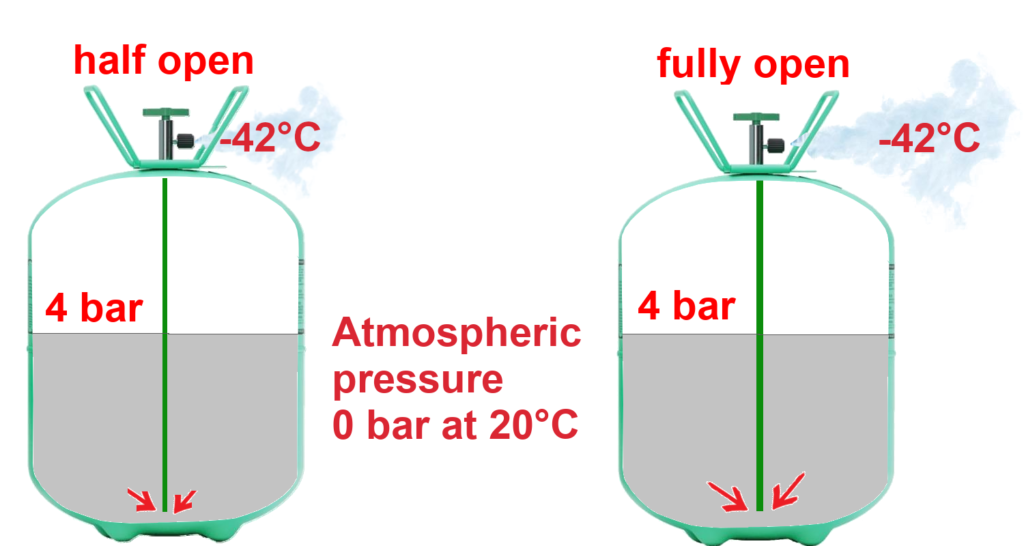
In contrast, look at another diagram. Again, for the same pressures in the cylinders, we can see quite easily that the more the valve is open, the greater the liquid flow from the valve.
In conclusion, the more the valve is opened and the higher the pressure in a cylinder, the greater the flow of liquid refrigerant escaping the cylinder.
But for the R22 liquid that emerges from the cylinder to evaporate, it must be able to absorb heat.
In this example, the R22 absorbs the heat it needs to evaporate from the surrounding air.
But since the R22 removes heat from the air. So the thermometer shows that the air must become colder if it falls to -42°C.
The vapourisation of R22 enables the chilling of the air.
But the more liquid escapes from the cylinder, the more heat the R22 will absorb to vapours, and the greater will be the chilling effect of the surrounding air.
In brief, then, the R22 contained in the cylinder can absorb heat that depends on
- The difference in pressure between the R22 and atmospheric pressure
- The extent of valve opening.
We can apply this knowledge to refrigeration systems. To cool the air, the R22 must vaporise, and the more R22 liquid that vapourises, the more we will cool the surrounding air.
The refrigerating capacity (also called the cooling capacity) varies according to the quantity of vaporised refrigerant. The greater the amount of R22 vapourised, the larger the refrigerating capacity.
Back to Basic!
Related
Read more: Fan wall
Read more: How to verify the percentage of outside air in an enclosure
Read more: BCA Part J5 Air-conditioning system control
Read more: Microbial Induced Corrosion (MIC) in Pipes
Read more: Is your kitchen exhaust system a fire hazard
Read more: What is coanda effect