What is Refrigeration and Air Conditioning
Refrigeration and Air Conditioning

Finding the heat exchangers
For this purpose, all refrigerators have two heat exchangers, the evaporator to absorb heat from inside and the condenser to discharge heat to the ambient.
The refrigerator has refrigerant instead of water. The same heat transfer principle applies to the fridge. It merely facilitates the heat exchange between the refrigerant and surrounding air.
The cold side heat exchanger is known as the evaporator. Do you know where the location of the evaporator? The evaporator is in the freezer compartment. The condenser is at the outside rear of the fridge. In the old-style fridges, you can see the condenser grille. When the refrigerator works correctly, this grille is warm to the touch.
Before we explain the working cycle of the refrigerator, let’s review a few fundamental principles.

What is heat
It is a sensation we experience when we feel the heat. For example, when we touch an object whose temperature differs from our hands, it might feel scorching, hot, warm, cool or cold, etc. When we touch an object, our brains compare the heat level in our body, hence temperature and the thing we feel.
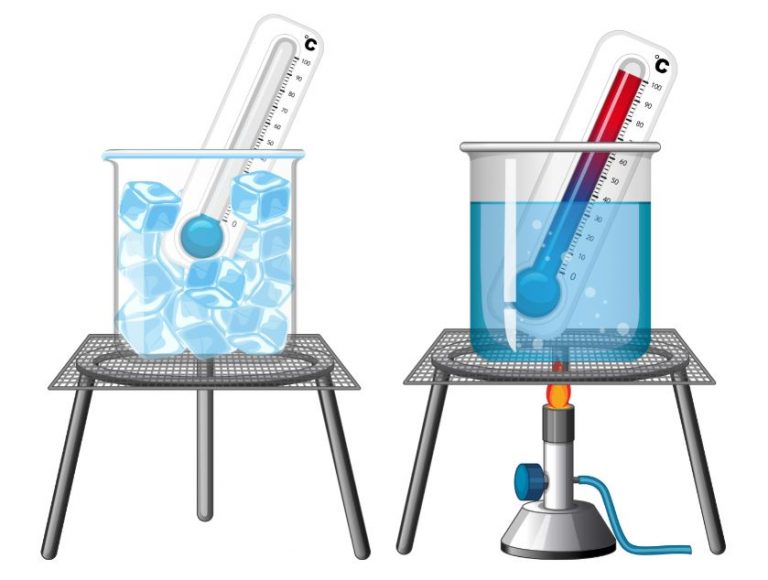
We denote 0°C on the °C scale to the melting ice temperature and 100°C correspondings to hot boiling water at sea level.
We use the thermometer to precisely determine heat levels, such as hot oil or acids in our bodies.
Heat Transfer
In our daily activities, we often heat and cool objects frequently. For example, we mix hot and cold water with a mixer tap to get a comfortable temperature for our showers.
We cause a thermal exchange by mixing the hot water at 45°C and cold water at 15°C for a resultant water temperature of 30°C.
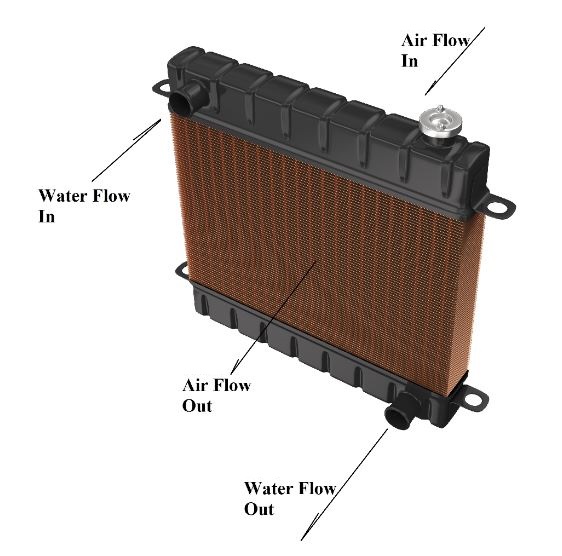
Thermal exchanges are limited to similar materials, and things that exchange heat don’t necessarily have to mix. So, for example, to heat a room, we can use a central heating radiator, inside which hot water is flowing.
This heat exchanger allows a heat transfer between the water in the heating system and the room’s ambient air.
Whilst the air becomes warmer, the hot water gets cooler as it passes through the radiator.

What do you think: does the water heat the air or vice versa?
Does Water Heat the Air or Vice Versa
We can verify this simply by measuring the hot water inlet pipe at the radiator’s top and its water outlet pipe.
We can deduce that heat transfer will occur when two bodies at different temperatures are in contact in observations earlier. We refer to this as a thermal exchange.
Heat always transfers from the hotter body to the colder. The air gains heat lost by the water. The heat the air gains equals that the water loses in a perfect thermal exchange.
The heat exchange is via the radiator as a heat exchanger.

Going back to the car radiator example, as water passes through the radiator, it loses heat. Its temperature falls from about 80°C to 60°C. The air gains heat lost by the water. It is why the temperature of the air increases across the radiator. We do not create heat or destroy. Only an exchange of heat takes place.
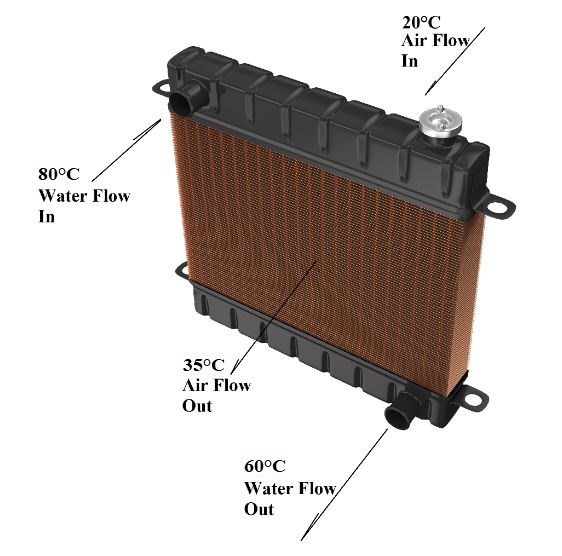
For example, if water arrives at 20°C, the ambient air temperature is also 20°C. Therefore, heat exchange is impossible since there is no temperature difference between the air and the water.
There can only be heat exchange between two bodies at different temperatures.
This fundamental principle also applies to our refrigerator. The cold fridge compartments should be at a lower temperature than our food.
Otherwise, there will be no heat exchange, and we won’t chill the food.
To further illustrate, for us to chill our food or drinks, we need to place them where the temperature is lower than its temperature, such as an esky with ice.
We will then cool the food, which is extracting heat from the food. The food gives up its heat to the surrounding ice. There is one problem.
The ice will warm up and melt as it absorbs the heat released by the food. However, the ice will not absorb any more heat when all ice melts.
So, we will not cool our food further when all the ice has melted. Then, we must put more ice into the esky. It seems straightforward, but where will we find more ice?
We can either get our ice from a glacier, which is not easy if you don’t live high up in the ice-capped mountains.
A freezer uses the same operating principles, but how does it work?
We will be able to understand the operation of air conditioning by first understanding the refrigerator.
Back to Basic!
Related
Read more: Fan wall
Read more: How to verify the percentage of outside air in an enclosure
Read more: BCA Part J5 Air-conditioning system control
Read more: Microbial Induced Corrosion (MIC) in Pipes
Read more: Is your kitchen exhaust system a fire hazard
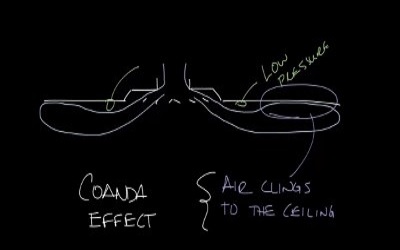
Read more: What is coanda effect