The Phenomenon of Condensation
The Phenomenon of Condensation
We’ve seen that to vaporise R22, that is, to change it from a liquid state into a gaseous state, we need to supply it with heat. So why, then, can’t we do the reverse? That is, why can’t we transform gas into a liquid state?
The reverse is possible, and this effect is called condensation. Before we go any further, let’s remind ourselves that the air surrounding us contains water vapour. This water vapour comes from the evaporation of the sea, lakes etc., and our respiration.

Where does the water that appears on the glass come from?
Note that we cannot see the air surrounding us or the water vapour. It is because the air and water vapour are both invisible gases to the naked eye. Nevertheless, we can also easily demonstrate the presence of water vapour in the air by blowing on a pair of spectacles.
In winter, the mist that comes from my mouth every time we breathe is water vapour! The water vapour we exhale becomes visible when the surrounding air temperature is low enough. In the same way, the misting of the windscreen of a car, the mist that we see on the kitchen windows in winter, the appearance of morning dew etc., are all phenomena that obey the laws of condensation.
This misting (condensation) occurs when water vapour comes into contact with a cold surface. For example, you use hot water in the bathroom when you use the bath or shower. The water vapour adds to the humidity of the surrounding air.
When the molecules of the relatively warm water vapour come into contact with the colder glass of a window or mirror, they immediately cool, and misting occurs.
Air is capable of holding large or small amounts of water vapour. It behaves like a sponge whose capacity for absorption varies according to how you squeeze it.
If you run a little water onto a sponge, it can absorb water.
If you squeeze the sponge afterwards, it releases the water as the squeezing” reduces its absorption capacity.
Air behaves a bit like a sponge, apart from its absorption capacity for water vapour doesn’t depend on squeezing. Instead, it depends on its temperature: the hotter the air is, the more capable it can contain water vapour; the colder it is, the less it can hold.

Why does the mist appear when air charged with water vapour comes into contact with sufficiently cold (glass, windscreen or other) surfaces?
It’s the same effect we noticed on a glass of chilled water in summer; it gets covered with moisture!
You will notice moisture on the outside of the glass and that the tablecloth is quite wet, Yet the glass isn’t leaking! How can we explain this?
The air and the water vapour cooled in contact with the cold surface. But the colder the air is, the less water vapour it can hold: if we cool it enough, the air will hold less and less water.
To avoid excessively scientific explanations, let’s say that when the temperature of the air has fallen sufficiently, it’s just like what happens when we squeeze a wet sponge hard enough.


The water vapour in the air starts to condense and ends up on the glass surface in mist, which is nothing but a fine layer of water in a liquid state.
The state of the table mat also confirms this, as it is quite wet, despite the glass not leaking.
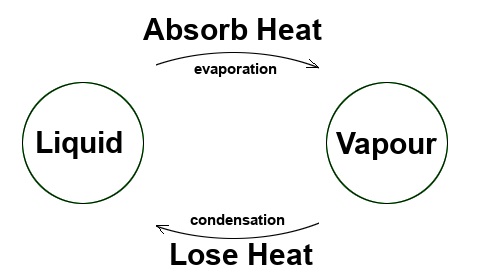
Thus, water vapour (a gas) changes into condensation or mist (a liquid) simply by cooling it.
We’re seeing an example of a change of physical state: A gas changing into a liquid.
To clarify this phenomenon, we can experiment.
Take a saucepan full of water and bring it to a boil. We know that after it reaches 100°C, the temperature remains constant. It is because water vaporises by passing from the liquid state to the vapour state.

Now, if you hold a plate at a distance of about ten or so centimetres above the saucepan, you’ll observe that the water vapour condenses freely on the plate.
Also, you’ll notice that the plate’s temperature increases considerably (TAKE CARE THAT YOU DON’T BURN YOURSELF!)

Given that the plate is neither in contact with the saucepan nor the flame, it must be the vapour that supplies heat to the plate as it condenses.
Despite being very simple to perform, this experiment nevertheless very important. It allows us to demonstrate that when water vapour is in contact with a cold surface, it condenses, and the cold surface becomes warmer.
The change from a vapour state to a liquid state is known as condensation.

To vaporise a liquid, we must heat it. To condense a vapour, we must cool it. Condensation is the opposite of evaporation. The vapour condenses on the plate, so there is a change of physical state, and it is the latent heat of condensation! The temperature remains constant when we consider latent heat during the state change.
When the vapour condenses, it gives up its heat at a constant temperature. The heat given out is also known as the latent heat of condensation.
Interestingly, the quantity of heat given up by 1 kg of water vapour during condensation is exactly equal to that needed for the vaporisation of 1 kg of water.
The phenomenon of condensation is, therefore, precisely the reverse of the phenomenon of evaporation.
- A liquid absorbs latent heat to allow it to vaporise and change into a gas: this is evaporation.
- A gas loses latent heat to condense and change into a liquid. It is condensation.
- Evaporation and condensation are changes of state that occur in a step at a constant temperature.
This phenomenon of condensation can help us improve the rudimentary refrigerator.
It will condense if we somehow recover the refrigerant vapour from the evaporator and place it in contact with a cold surface. That is, it will change once more if to a liquid.
So we now have a big problem; to condense, gaseous R22 should be in contact with a body much colder than itself, but it’s 20°C in the kitchen, and the gas at the evaporator outlet is at -42°C.
It seems impossible to condense R22 vapour then, unless we heat it beforehand, is it?

Back to Basic!
Related
Read more: Fan wall
Read more: How to verify the percentage of outside air in an enclosure
Read more: BCA Part J5 Air-conditioning system control
Read more: Microbial Induced Corrosion (MIC) in Pipes
Read more: Is your kitchen exhaust system a fire hazard
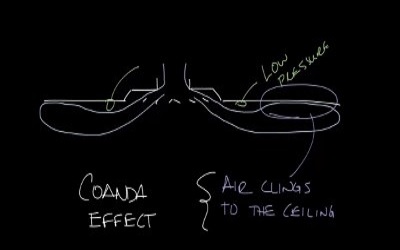
Read more: What is coanda effect