The Role of the Expansion Device (TXV and EXV)
The Role of the Expansion Device (TXV and EXV)
In the instance of a bicycle pump experiment, you will notice the air heats as it escapes from the piston past your thumb, which is partially blocking the outlet.
If you lift your thumb, the air compresses much less, and the temperature drops. You can then operate the ‘pump’ with little difficulty, as the air pumps out at low pressure.
The converse is also true. The more you block the outlet with your thumb, the more you notice the temperature rise, making it more difficult to operate the pump.
In effect, the more you obstruct the outlet orifice, the more the pressure increases and the temperature rises.
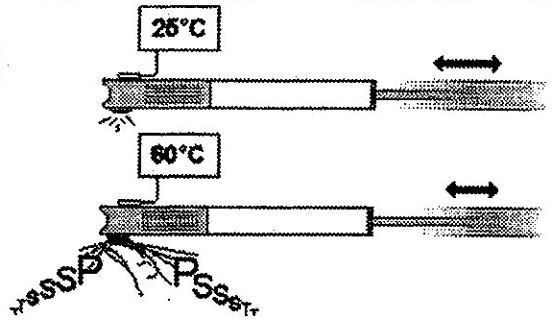
In effect, the more you obstruct the outlet orifice, the more the pressure increases and the temperature rises.
It’s the same for a fridge compressor: the higher the pressure becomes, the greater the temperature of the gas it pumps out.
We saw that the condensation of R22 vapour was only possible if the vapour temperature exceeded the environment. So with an ambient temperature of 20°C, we must raise the temperature of the R22 to something greater than 20°C.
Pressure-Temperature Relationship
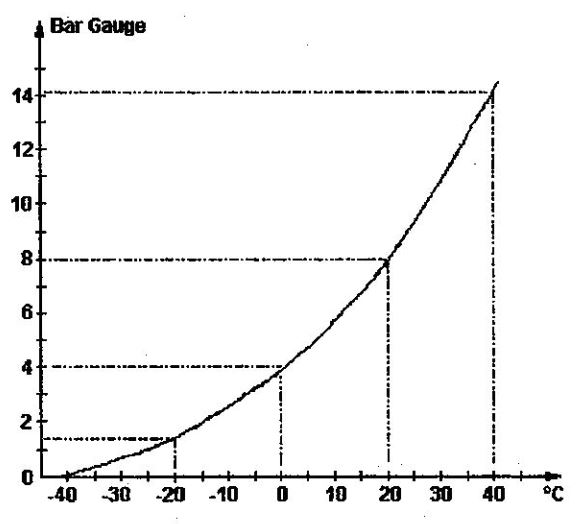
The pressure-temperature relationship for R22 shows that at 20°C, condensation (or evaporation) occurs at a pressure of about 8 bar.
We can conclude that to condense R22 vapour from the evaporator at -42°C with the surrounding air at 20°C. We must not only increase the temperature of the vapour to above 20°C but also increase its pressure to something over 8 bar!
The greater the outlet pressure of the compressor is, the higher the temperature of the R22 vapour will be, and the easier it will be to cool and condense.
Remember that the more we block the outlet of the bicycle pump, the higher the resulting pressure and temperature.
The air from the pump is very hot and can only get colder.
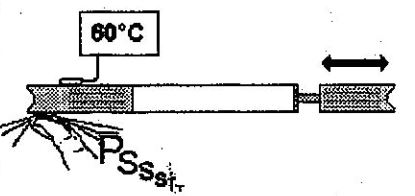
In a way, if we want to cool gaseous R22 to make it condense. We must increase the temperature and pressure inside the condenser.
Using the example of the bicycle pump, we may partially obstruct the condenser outlet with our ‘thumb’!
In this way, the refrigerant drawn from the LP evaporator, then compressed by the compressor, will be pumped out at HP and maintained at high pressure in the condenser, similar to the ‘thumb’ effect that partially obstructs its outlet.
Since the refrigerant is at HP in the condenser (well above 8 bar), its temperature will be more than 20°C. The air at 20°C in the kitchen will easily cool the hot R22 vapour and allow it to condense.
As the R22 vapour condenses, liquid R22 will emerge from the condenser. But you may recall that liquid R22 vaporises at -42°C at atmospheric pressure. We must use something other than a ‘thumb’ to make a partial obstruction at the outlet of the condenser, or we’ll risk burning ourselves quite severely!
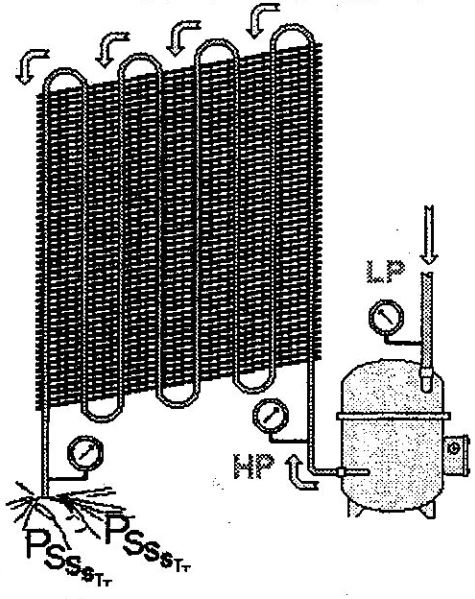
Instead of our thumb at the condenser outlet, we install, for example, a restrictor or a special valve. This valve can open to varying degrees.
The compression of gas causes an increase in its pressure and also an increase in its temperature.
This valve allows liquid refrigerant to pass from a high HP pressure value to a much lower LP pressure (in our example, the pressure at the outlet is equal to atmospheric). It causes a significant pressure drop.
In the jargon used by refrigeration professionals, we say that a fluid that experiences a large pressure drop undergoes an expansion. In your opinion, what should we call this valve?
We naturally call this device that causes an expansion (a large pressure drop) an “expansion valve”. So the device that causes a large reduction in pressure from HP to LP is the expansion device.
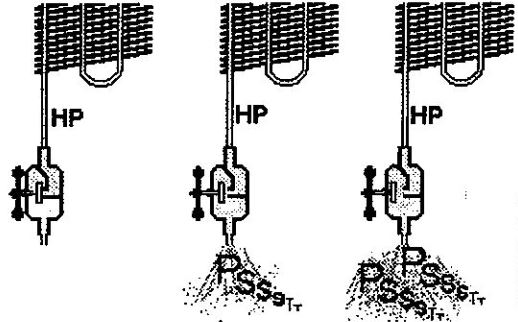
Our valve here functions as a manually controlled expansion device. We rarely use such expansion devices nowadays, and numerous other fully automatic expansion devices exist. For example, the device used in a refrigerator isn’t a valve. Do you know whereabouts is the expansion device in your fridge?
Whatever technology we use, the expansion ‘valve’ is situated somewhere along the tube that emerges from the condenser. In small, highly mass-produced equipment (refrigerators, air- conditioners etc.), the market is highly competitive, and the price of the equipment is a critical feature.
For economic reasons, manufacturers install expansion devices with the lowest possible cost. These are usually lengths of copper tubes of very small diameter through which liquid refrigerant flows with difficulty. It causes a “restriction”, just as our thumb did earlier.
We call the narrow tube (1) a “capillary” or capillary expansion device.
At the condenser outlet, you should also have been able to see the filter (2). Its role is to trap any possible impurities (such as copper filing, bits of abrasive or brazing materials) which could block the very fine interior diameter of the capillary expansion device.
The other point of interest is that the capillary tube passes inside the compressor inlet pipework. We assure the integrity of the capillary’s entry point by brazing the access point (3). Finally, note that the refrigerant flows independently in the two tubes (4), and no mixing is possible. This feature is simply a technical option taken by the manufacturer.
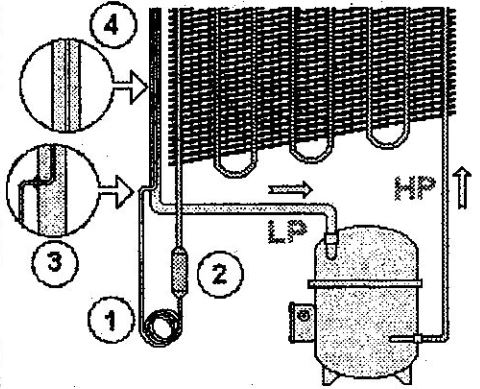
Before continuing with the capillary expansion ‘valve’, look closely at the above diagram, and think about these two questions: the physical state (vapour or liquid) and the pressure (LP or HP) of the refrigerant.
- At the condenser inlet?
- At the inlet to the capillary?
The compressor draws LP vapour (5), and after being compressed in the cylinder, it discharges refrigerant towards the condenser (6). This very hot HP vapour emerges from the compressor and enters the condenser.
Since the R22 vapour is much hotter than the surrounding air, it condenses by giving up heat to the air in the kitchen. As a result, we will find high-pressure R22 liquid at the outlet of the condenser and the inlet to the capillary (7).
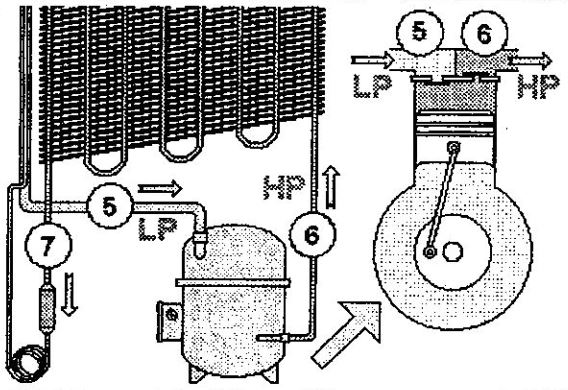
We can improve our knowledge of the capillary expansion device a little. But first, we’ll often encounter this type of expansion device in our small refrigeration systems. Although it is a very simple technology (it is simply a length of tubes of small diameter), the capillary action is the same as that of the valve seen earlier: It creates resistance, or opposition, to the refrigerant flow.
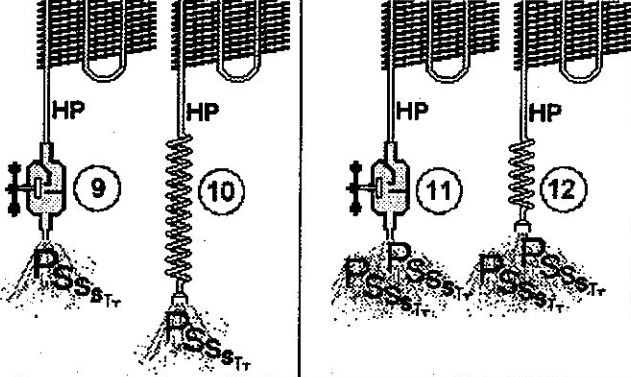
In the case of the valve, the resistance depends on its opening aperture. If it’s almost closed, the valve will only allow minimum flow (9). However, the valve enables maximum flow (11) when fully opened. The flow is a function valve opening, that is, of the resistance of the valve to the refrigerant flow.
In contrast, the resistance of the capillary depends on its length. A fairly long capillary (10) provides a high degree of resistance to the refrigerant flow, just like a nearly closed valve. Conversely, if we shorten the capillary (12), its resistance decreases: the refrigerant can flow easily, just as it would through a fully opened valve.
Can you see anything else that could affect the resistance of a capillary?
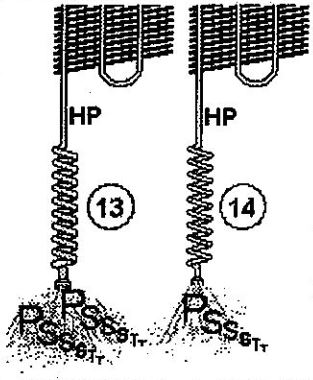
Another critical influence on the capillary’s resistance is the tube’s internal diameter. The capillary in (13) has a larger diameter than the capillary in (14). Therefore, although both capillaries are of the same length, the flow through (13) (large diameter) is much greater than the flow through (14) (small diameter).
Thus, the smaller the capillary tube’s internal diameter is, the greater the resistance to the passage of refrigerant and the smaller the flow.
In summary, the resistance of the capillary depends on its length and diameter. However, once installed, the dimensions of the capillary won’t change, and it has a fixed resistance to refrigerant flow.
A capillary is like a valve stuck in a position defined by the manufacturer!
Never forget that a capillary expansion device is a valve that’s absolutely stuck. It is specified by the manufacturer depending on the cooling capacity, the quantity of refrigerant in the equipment, the designed operating conditions etc. If any one of these values changes, the capillary won’t give the required performance. In effect, the capillary acts as an interface between HP and LP: The expansion device is vital!
Without an expansion device, there can be no compression, heating, condensation, liquid refrigerant, and evaporation!
Now that we know that we have liquid refrigerant arriving at the inlet to the expansion device, we’ll try to determine precisely the refrigerant’s physical state at its outlet.
So that we can obtain a better understanding of what happens at the expansion device, we’ll perform a straightforward experiment together.
- Take a can of hair spray or pressurised aerosol deodorant. Shake it whilst listening carefully to the noise it makes inside, and then try to estimate its temperature.
- Now press the spray head and send a jet of product onto the inside of your other hand. Look at what’s on your hand and wait for a short while
- What sensation could you feel on the palm of your hand: cold or heat? What can you conclude from this?
Inside a can like this, there is a mixture of liquid and vapour. You can tell that this is so from the noise that you hear.
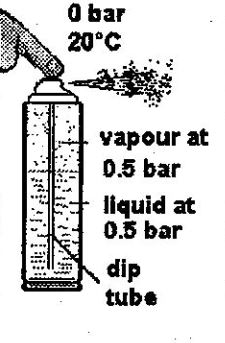
When you hold the can in your hand, you can feel that it is at ambient temperature, at about twenty degrees. Let’s say that at 20°C, the pressure inside the can is, for example, 0.5 bar. That is, slightly more than atmospheric pressure (which, you will remember, corresponds to 0 bar).
Then, some product is discharged from the can when you press the spray. It proves that the pressure inside the can is greater than atmospheric pressure. Furthermore, you observe that the product is liquid, so this must be at the bottom of the can and have a dip tube.
What do you observe when you spray a little of the product onto your palm: Firstly, you can distinctly see that it’s in liquid form. After waiting a little while, you carefully observe what happens to the liquid in your hand. You see that the liquid is gradually disappearing and that simultaneously you can feel a cooling sensation on the palm of your hand. What is happening?
As it passes through the small orifice of the spray, the pressure of the liquid falls from 0.5 bar (inside the can) to atmospheric pressure (that is, 0 bar).
But do you remember that a device that causes an expansion (a fall in pressure) is called an expansion device? So the spray head of the aerosol can act like a mini-expansion device.
Are you starting to understand the cooling sensation that you felt?
Remember that when we released R22 at 20°C and 8 bar pressure from a cylinder, it expanded until atmospheric pressure (the surroundings were at 20°C and 0 bar). The liquid vaporises by absorbing heat from the surrounding air, and from your hand, remember that liquid R22 evaporates at -42°C at atmospheric pressure and THAT YOU RISK SEVERE BURNS. WARNING DONT”T DO THIS WITH REFRIGERANT!
As it emerged from the aerosol can, the liquid at 20°C and 0.5 bar expanded until it was at atmospheric pressure (the surroundings were at 20°C and 0 bar) exactly as the R22 did (but fortunately, manufacturers put liquids into their sprays that vaporise well above 0°C). However, in order to evaporate, all liquids must absorb heat from somewhere. It is why you feel a cooling sensation on the palm of your hand as the liquid gradually absorbs heat from it to vaporise and dissipate into the atmosphere.
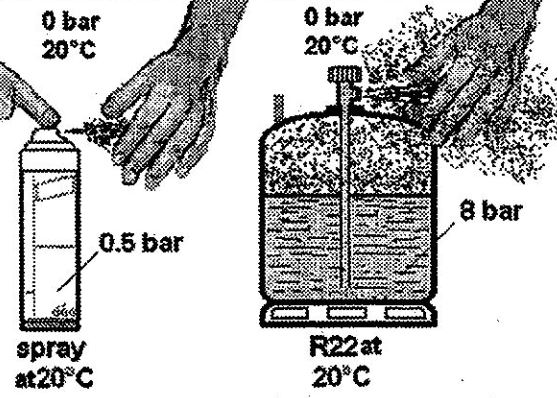
In these two examples, the spray head of the aerosol can, like the valve of the R22 cylinder, acted as an expansion device. By creating a pressure drop of 0.5 bar for the aerosol and 8 bar for the cylinder valve, they caused the evaporation of the liquid. In our refrigeration system, this role of the expansion device falls to the capillary. We know that the resistance of the capillary depends on its length and diameter.
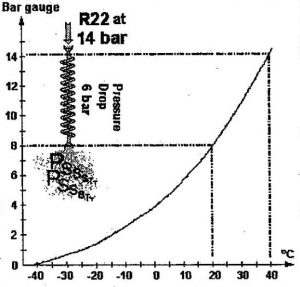
Let’s assume the R22 liquid from the condenser arrives at the inlet to the capillary at 14 bar. Then, it would correspond to a condensation temperature of 40°C, as is shown by the pressure-temperature curve for R22.
If we design the capillary to create a pressure drop of 6 bar, the liquid at its outlet will be at 8 bar and vaporise at 20°C: do you think this will be sufficient to chill our foodstuffs?
On the other hand, if a manufacturer has chosen a longer capillary, which creates a pressure drop of 10 bar (instead of the previous 6 bar), the liquid will be at 4 bar at the outlet. As the pressure-temperature relationship for R22 shows, the liquid will vaporise at 0°C, which would already seem more satisfactory for chilling our food.
If the capillary expansion device is longer still (or of smaller diameter), the pressure drop will be even larger, and the evaporation temperature will be even lower. Thus, if we want the liquid to evaporate at about -20°C (which corresponds to a pressure of 1.5 bar), we need to select a capillary that generates a pressure drop of 14 – 1.5 = 12.5 bar.
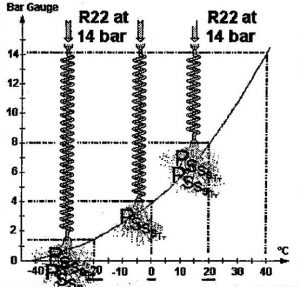
Thus, depending on the operating conditions required and the type of equipment (fridge, air conditioner, freezer), the manufacturer can determine in advance the pressure drop that a capillary must generate.
So, we’ve seen that the compression of a gas has the effect of increasing its pressure and temperature. In contrast, the expansion of a liquid has the effect of reducing its pressure and temperature.
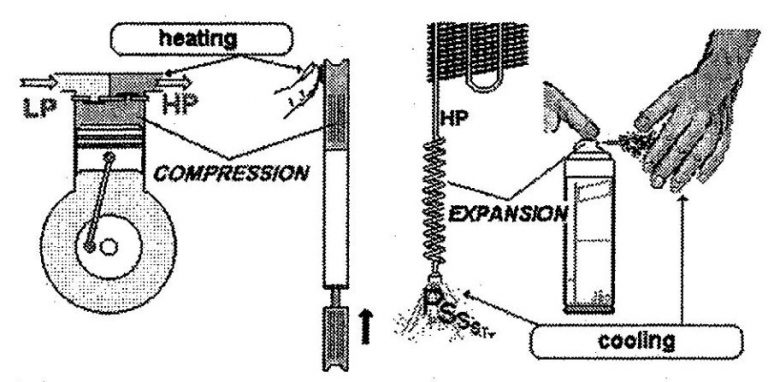
Expansion produces precisely the opposite effects of compression.
Compression = Increases in pressure and temperature
Expansion = decreases in pressure and temperature
We’ve now covered some of the operating principles of refrigeration systems. But before we continue, let’s revise the principal rules we should remember.
- There can only be heat transfer between two bodies if those two are at different temperatures.
- Heat always flows from a hotter body to a colder one.
- The change from the liquid state to the vapour state is called vapourisation.
- When a liquid evaporates, it absorbs heat, but its temperature remains constant. The heat absorbed by the liquid in this way is called the latent heat of vapourisation.
- The evaporation temperature and the evaporation pressure always vary in the same direction. It increases or diminishes together.
- We call the cold heat exchanger, where the refrigerant vaporises by absorbing heat, the evaporator.
- The change from the vapour state to the liquid state is called condensation.
- As a vapour condenses, it gives out its heat at a constant temperature. The heat is the latent heat of condensation.
- The heat exchanger where the refrigerant condenses is called the condenser. It is this heat exchanger that gives out heat.
- The compression of a gas has the effect of increasing its pressure and its temperature.
- The compressor is the equipment that allows us to compress the refrigerant.
- The device that causes a large pressure drop (the change from HP to LP) is the expansion device.
- The expansion device is the interface between HP and LP. Without the expansion device, there can be no compression, heating, condensation, liquid refrigerant, and evaporation. No expansion device, no refrigeration system!
If you want to understand how an air- conditioner works, you must understand these rules precisely!
Back to Basic!
Related
Read more: Fan wall
Read more: How to verify the percentage of outside air in an enclosure
Read more: BCA Part J5 Air-conditioning system control
Read more: Microbial Induced Corrosion (MIC) in Pipes
Read more: Is your kitchen exhaust system a fire hazard
Read more: What is coanda effect