What is Boiling
What is Boiling

Latent Heat of Vapourisation





When a liquid evaporates, it will absorb heat. As a result, its temperature will remain constant. The heat absorbed is called the latent heat of vapourisation.
These experiments demonstrate that when water changes from liquid to vapour, it absorbs heat without changing its temperature.
At normal atmospheric pressure, water vaporisation always takes place at 100°C.
Latent Heat of Fusion
What do you think will happen?
Despite adding heat, the water temperature remains stable at 0°C, but the ice melts and changes its state to liquid. This phenomenon is called the fusion of ice.

As we add heat, the water temperature will rise as soon as there is no ice left.
These experiments allow us to observe the fundamental phenomena critical to understanding air conditioning systems’ refrigeration cycle.
Water changes from the solid state to the liquid state at a constant temperature of 0°C by absorbing heat. This heat is known as the Latent Heat of Fusion.
When we add heat, and there is no increase in temperature, but it causes a change in its physical state, we refer to it as latent heat.
On the other hand, when the temperature changes by adding heat, for example, when we heat water from 20° to 40°C, we say it is due to sensible heat. So, in a way, we can sense the temperature change!
We must always remember that evaporation needs energy (heat). And more generally, liquid can not evaporate, nor does ice melt without adding heat.

The liquid absorbs enormous amounts of energy as it evaporates. For example, our experiment shows that liquid water requires 5.4 times more energy to change into vapour at a constant temperature of 100°C than to raise its temperature from 0°C to 100°C.

Of course, once it reaches 100°C, the temperature stabilises; this is the beginning of a second step, the one we have already seen.
This experiment has allowed us to observe some fundamental phenomena.

Water changes from solid to liquid at a constant temperature (0°C) by absorbing heat. This heat is known as the Latent Heat of Fusion.
Water changes from the liquid state to the Vapour State at a constant temperature (100°C) by absorbing heat. This heat is known as the Latent Heat of Vaporisation.
Latent heat is the heat needed to cause the change in the physical state of a body at a constant temperature.
On the other hand, when the temperature changes by adding heat, for example, when we heat water from 20° to 40°C, we say it is due to sensible heat. So, in a way, we can sense the temperature change!
We must always remember that evaporation needs energy (heat). And more generally, liquid can not evaporate, nor does ice melt without adding heat.
What heat is required to transform 1 kg of pure ice at -50°C into 1 kg water vapour at 150°C?
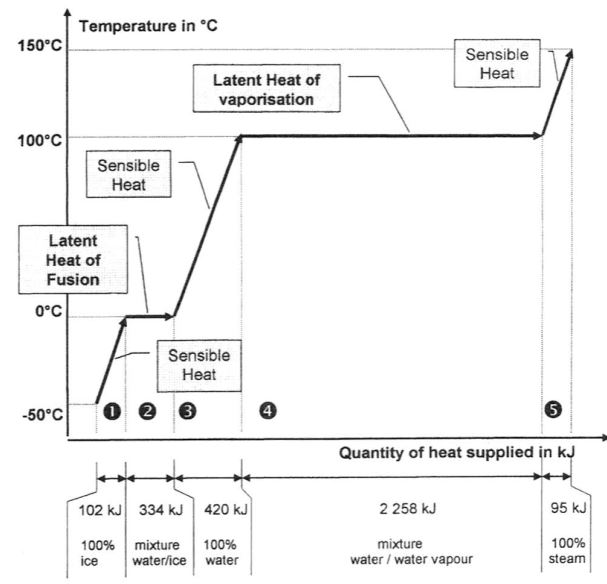
The unit of heat (energy) is in kilojoules (kJ). These values are only for background, don’t worry if they mean little now. Just note that the vapourisation step lasts seven times longer than the fusion step with the same heat source and the same amount of water.
The liquid absorbs enormous amounts of energy as it evaporates. For example, our experiment shows that liquid requires 5.4 times more energy in vapour at a constant temperature of 100°C than raising its temperature from 0°C to 100°C.
These are the laws of natural physics and are the basis of the refrigeration cycle.
Back to Basic!
Related
Read more: Fan wall
Read more: How to verify the percentage of outside air in an enclosure
Read more: BCA Part J5 Air-conditioning system control
Read more: Microbial Induced Corrosion (MIC) in Pipes
Read more: Is your kitchen exhaust system a fire hazard
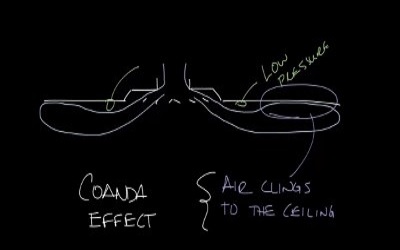
Read more: What is coanda effect